May 02, 2024 Industry news
NHS Supply Chain's new policy aims to improve the traceability and safety of medical devices and clinical consumables.
About the policy
NHS Supply Chain’s recently announced the launch of a new policy on data standards for supplier product coding. The policy highlights GS1 standards as the preferred data coding standard for products – notably the GS1 Global Trade Item Number (GTIN).
The purpose is to ensure medical devices and clinical consumables, that are supplied to the NHS, use globally recognised coding standards for unique identification. This will enable products and devices to be effectively tracked and traced throughout the supply chain from the point of manufacture to the point of care.

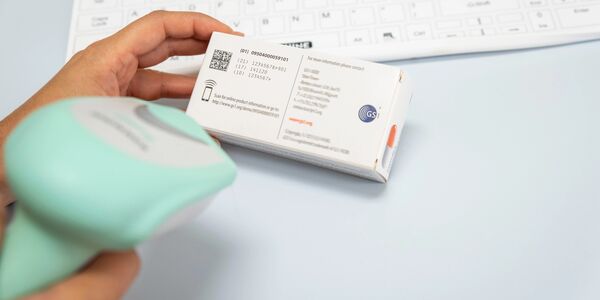
The policy also insists that medical devices carry barcode labels which are compliant with Unique Device Identification (UDI) requirements for global regulations. This means that the barcodes must contain scannable information about the production of the device, such as the expiry date and the serial or lot number.
Clinical consumable products should also carry a barcode label that can be scanned for inventory management purposes.
For details on what you need to do to comply, read NHS Supply Chain’s guidance document on supplier requirements.
Benefits of the policy
The new policy will enable the NHS to collect and use data on medical devices and clinical consumables in a consistent and standardised way. This will help NHS Supply Chain to improve the quality of its critical product data and increase the number of GTINs for medical products in its product catalogue.

Standardised coding will enable clinicians to improve the traceability of products and medical devices used in each episode of a patient’s care in order to better monitor the performance of each product and the outcomes for each patient.
This approach will also help to reduce waste and inefficiency across the NHS as inventory management systems will be able to better manage stock and usage levels of products. Additionally, this will support data capture for the Federated Data Platform to provide system-wide visibility of inventory data.
Alignment with national strategies
The new policy aligns with existing strategies and policies which also rely on unique and standardised product coding, including:
- NHS Supply Chain’s In-Trust Inventory Management Systems (IMS) deployment programme which centres on using product information for inventory management to support scanning at the point of care
- NHS England's Scan4Safety initiative which uses point-of-care scanning to capture product information for patient safety
- NHS England’s Outcomes and Registries Programme which requires accurate product information for national registries to monitor patient outcomes at scale
- NHS England’s Strategic Framework for NHS Commercial which requires accurate product information to strengthen procurement approaches and drive transparency across the system
- The Department of Health and Social Care’s (DHSC) MedTech Strategy which aims to support MedTech procurement to ensure the right product, is acquired for the right price, and delivered to the right place to support resilience and continuity of supply. With the development of the National Equipment Tracking Information System (NETIS) as part of this, a systematic and proactive approach to data gathering on equipment is also possible, so timely decisions can be made routinely and flexibly.
Why GS1 standards?
GS1 standards for unique identification are system and device agnostic, therefore facilitating interoperability between systems and organisations to support data exchange and integration.
GS1 is also authorised as an issuing entity for UDI which means suppliers and manufacturers can use GS1 standards to comply with international medical device regulations. More than 95% of medical devices* already used GTINs for UDI.
NHS Supply Chain have selected GS1 standards as the preferred standards for product coding and UDI as they are widely recognised and accepted internationally.
* In a survey conducted with Portsmouth Hospitals University NHS Trust and GS1 Healthcare in which 2400+ medical devices were reviewed to gauge adoption levels.