January 21, 2026 Industry news
On 1 January 2025, new medicines rules under the Windsor Framework came into effect across the UK. The Framework was introduced to replace parts of the Northern Ireland Protocol and simplify how medicines move between Great Britain and Northern Ireland. Previously, Northern Ireland was required to follow the EU Falsified Medicines Directive (FMD), even after the rest of the UK left the EU.
FMD was an EU safety regulation designed to prevent falsified medicines entering the supply chain. It required every prescription medicine pack to carry a unique serialised 2D GS1 DataMatrix and a tamper‑evident seal, enabling authenticity, batch and expiry information to be verified before dispensing.
With FMD no longer applying anywhere in the UK, manufacturers supplying UK‑only packs are no longer required to include the FMD‑mandated GS1 DataMatrix 2D barcode. As a result, more medicines are now arriving in NHS organisations without the GS1 DataMatrix and in some cases without any product identifier at all, including the simple linear barcode that support accurate scanning, traceability and medicines safety. While regulatory alignment was the goal, the practical impact on patient care and safety is becoming increasingly clear.
Growing evidence of safety risks
Hospitals and pharmacy professionals have raised increasing concerns about the quality and consistency of barcode information on some UK‑only medicine packs. According to the MHRA Inspectorate, recent issues reported across the system include:

- Global Trade Item Numbers (GTINs) that scan as the wrong medicinal product
- Unreadable or missing GS1 DataMatrix codes, making products unscannable
- Incorrectly formatted DataMatrix data causing automated systems to reject stock
- Uncontrolled GTIN reuse leading to product confusion
- Barcode errors resulting in stock being quarantined or requiring manual rechecking
These errors directly impact patient safety. Missing or faulty barcodes prevent clinicians and pharmacy teams from capturing product data accurately, and they undermine the digital safeguards that scanning is designed to provide. The MHRA has highlighted delays, rework and manual checks introduced by these issues, noting that they have already caused avoidable harm in some cases.
Impact on scanning workflows and medicines management
Barcode issues affect the entire medicines management process, from supply and dispensing to administration.
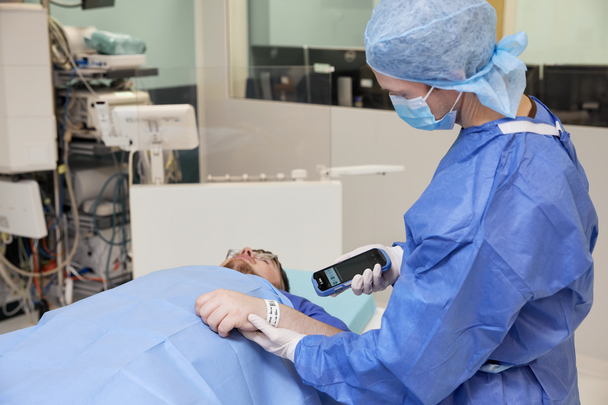
Point of care scanning has become a cornerstone of safe medicines practice in many healthcare providers across the UK. By scanning the GS1 DataMatrix 2D barcode at the bedside, clinicians can confirm the five rights of medicines administration (the right patient, drug, dose, route and time) within seconds.
Closed‑loop medication administration (CLMA) uses digital technologies to provide additional verification of the five rights. It forms a key component of closed‑loop medicines management, which relies on integrated digital systems to support and safeguard every stage of the medicines process.
When a barcode is missing or cannot be scanned, this safety loop breaks and staff must revert to manual checks. This increases the risk of errors and extends the time required to complete tasks. Many medicines look alike, sometimes differing only in strength or presentation, so manual verification increases the likelihood of error.
The impact extends beyond the point of care. Barcode failures can disrupt:
- Automated dispensing inventory management
- Electronic patient records
- Product recalls
- Incident investigations
- Audit and reporting
- Clinical confidence in scanning systems
Every failure results in workarounds that slow down workflows, increase cognitive load and reduce the reliability of systems designed to support safe, efficient care.
Why GS1 standards continue to matter
GS1 standards provide a globally recognised system for identifying medicines consistently and reliably. A GS1 DataMatrix encodes essential information — such as the GTIN, batch number and expiry date — in a format that clinical and supply chain systems can read instantly.

The importance of consistent product identification is also reflected across wider NHS policy frameworks. GS1 standards underpin national initiatives including the NHS eProcurement Strategy, the NHS England strategic commercial framework and Scan4Safety. The Patient Safety Commissioner for England has emphasised that accurate barcoding is critical to preventing potentially fatal medication errors, while recent MHRA Inspectorate findings highlight the real‑world impact of missing, incorrect or poorly formatted barcodes on patient safety and clinical workflows.
NHS programmes such as Scan4Safety rely on GS1 standards to track patients, products and places across care pathways. By using GS1 barcodes to capture data accurately at each step, Scan4Safety improves safety, reduces variation and supports more efficient, transparent clinical workflows.
Although the Windsor Framework removes the legal requirement for EU serialisation on UK‑only packs, the clinical and operational value of GS1‑compliant DataMatrix barcodes remains unchanged. Without them, the NHS loses critical safety information, and the digital systems that support CLMA, stock control and recall management cannot function effectively.
Healthcare leaders, including the UK’s chief pharmaceutical officers, have already encouraged manufacturers to continue applying 2D barcodes across all UK packs to maintain medicines safety and support the NHS’s digital ambitions.
Supporting safer care through accurate data
The adoption of digital systems and technologies in medicines management has already delivered significant benefits across the NHS, from improving the accuracy of documentation to reducing medication administration errors. Barcode data captured at the point of care supports clinical decision‑making, strengthens audit trails and enables more effective recall responses.
But these gains rely entirely on the data printed on each medicine pack. If a barcode is missing or inaccurate, the NHS cannot fully realise the benefits of digital medicines management or maintain the high level of safety that CLMA provides.
To protect these advances, manufacturers supplying medicines to the UK should ensure their packs continue to carry GS1‑compliant DataMatrix barcodes that meet global best practice. Consistent, accurate data now will help protect patients and support the NHS as it continues its digital transformation.
A collective effort to protect patients
The Windsor Framework is changing how medicines are labelled, but it has also highlighted how essential accurate barcode data has become across modern healthcare. Ensuring medicines remain traceable, verifiable and safe requires coordinated action across the entire system, from manufacturers and distributors to regulators and NHS teams.
GS1 UK will continue working closely with suppliers, NHS organisations and policymakers to promote consistency in the application of GS1 standards. Reliable, high‑quality barcode data is crucial to safeguarding patients, supporting clinical staff and enabling the NHS to build a safer, more digitally enabled future.